The content of this presentation is provided to an audience of international healthcare professionals from around the world for scientific discussion and scientific exchange. Bayer does not recommend the use of any drug substance outside of its approved indications.
At the moment of publication, the compound presented is solely approved for use in the US by the US Food and Drug Administration (FDA) in chronic kidney disease (CKD) associated with type 2 diabetes (T2D). The compound is still being investigated for uses that have not been approved by the European Medicines Agency (EMA) and other health authorities.
Presentations of the Bayer Industry Symposium at the EASD 57th Annual Meeting 2021
Thank you for your interest! See below to watch the session recording of the EASD symposium video.
Should you wish to receive further updates on the materials featured here then please follow this link to submit your details.
Finerenone

Wednesday 29 September, 2021 | 18:30 - 18:35 CEST | On Demand
New perspectives and interdisciplinary insights on the benefits of MR antagonism in CKD and T2D
Session type: Industry Symposium Faculty: Paola Fioretto, Samy Hadjadj, Roland Schmieder
Should you wish to receive further updates on the materials featured here then please follow this link to submit your details.
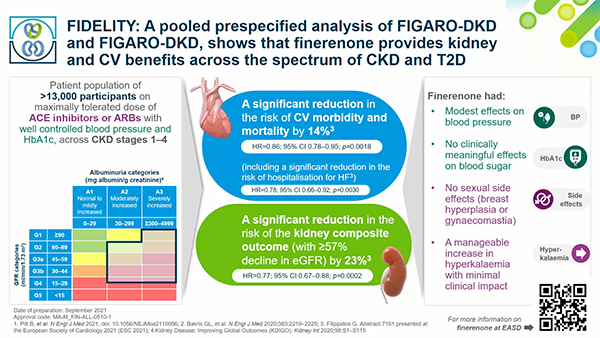
Wednesday 29 September, 2021 | 19:45 - 20:45 CEST | On Demand
EASD 2021 Meet the Experts Session
Session type: Industry Symposium Faculty: Paola Fioretto, Samy Hadjadj, Roland Schmieder
Should you wish to receive further updates on the materials featured here then please follow this link to submit your details.
More Information
For more information regarding Finerenone visit us by clicking the link below
The compound presented is approved for use by the US Food and Drug Administration (FDA) in chronic kidney disease (CKD) associated with type 2 diabetes (T2D) but is investigational or being investigated for uses that have not been approved by the European Medicines Agency (EMA) and other health authorities.
Date accessed: 1 September, 2021
MA-M_FIN-ALL-0525-1




